Pediatric Hematopoietic Stem Cell Transplant Fellowship Curriculum
The following table summarizes the time commitment allotted for each rotation to meet the specific goals and objectives on the fellowship:
| Rotation | Time |
| Inpatient Service (IP) | 20 weeks |
| Outpatient Service (OP) | 16 weeks* |
| Research (R) | 12 weeks |
| Vacation (PTO) | 4 weeks |
| Total | 52 weeks |
*2 weeks of electives may be taken if interest is made. Electives include: Radiation Oncology, Transfusion Medicine, HLA Lab, or Stem Cell Lab
Additionally, all fellows would have a weekly continuity clinic, which will consist of a variety of patients accrued over the course of the year.
All fellows will be expected to participate in the Hematology/Oncology education opportunities as well as develop a clinical research project under close mentorship by a faculty member.
Fellows are expected to work one weekend during each 4-week block.
Sample Block Schedule for Pediatric HSCT Fellowship:
| Block 1 | Block 2 | Block 3 | Block 4 | Block 5 | Block 6 | Block 7 | Block 8 | Block 9 | Block 10 | Block 11 | Block 12 | Block 13 | |
| Week 1 | IP | IP | IP | PTO | IP | OP | OP | OP | OP | IP | IP | PTO | OP |
| Week 2 | OP | OP | IP | IP | IP | IP | IP | IP | IP | IP | IP | IP | R |
| Week 3 | R | R | OP | R | R | IP | IP | R | R | R | R | IP | IP |
| Week 4 | R | R | OP | OP | OP | OP | OP | PTO | PTO | OP | OP | OP | R |
Schedules can be individualized based on particular experience/interest of specific candidates
Additionally, our program provides opportunities to collaborate with colleagues at Mayo Clinic whom Phoenix Children's shares a joint-FACT accreditation. At Phoenix Children's, we lead the state of Arizona in the number of transplants performed annually in pediatrics and were the first center in the state to offer chimeric antigen receptor T-cell (CAR-T) therapy.
Procedures
| Procedures | Number of Procedures |
| Bone Marrow Harvests | Expect 5 per year |
| Bone Marrow Aspirates/ Biopsies | Expect 5 per year |
| Lumbar Punctures | Expect 5 per year |
| Stem Cell Transplants/Cellular Therapy Administration | Expect Fellow to oversee 15 during the year |
All Bone Marrow Transplant (BMT) fellows will be required to do the following procedures: In 2019, the BMT Program at Phoenix Children’s performed the following: 47 hematopoietic stem cell transplants (HSCT) and 8 chimeric antigen receptor T-cell (CAR-T) cellular therapies. Within these 47 HSCT, 8 were from sibling donor bone marrow harvests (Figure 1).
Figure 1:
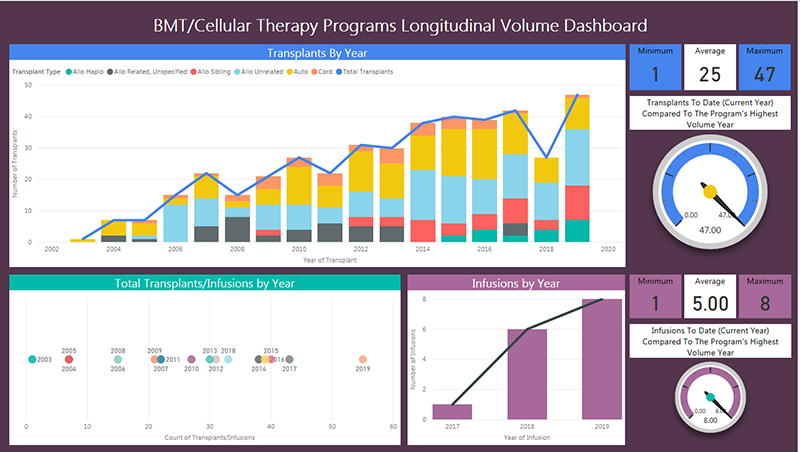
All malignant patients and some non-malignant patients require bone marrow aspirates and/or biopsies following HSCT. High risk malignant patients require a total of 6 bone marrow aspirates within the first-year post HSCT. Additionally, patients with central nervous system disease prior to undergoing HSCT require screening lumbar punctures following HSCT (monthly for 3-6 months). This will provide plenty of opportunity for a training fellow to practice and sign off on procedures. Please see image below for full specifications from the BMT/Cellular therapy volume.
